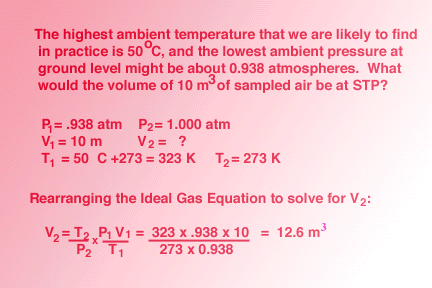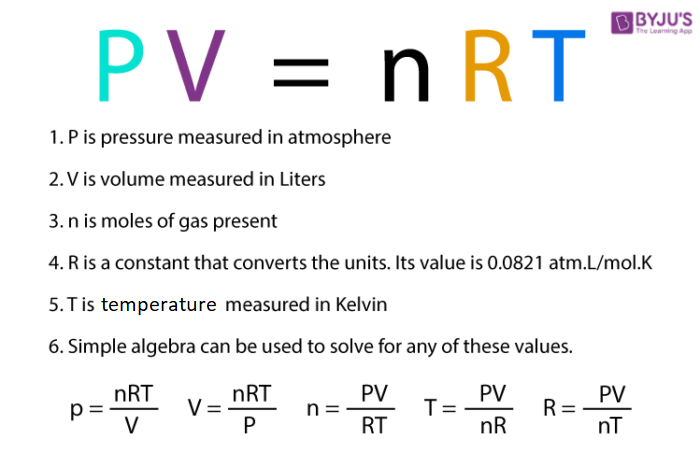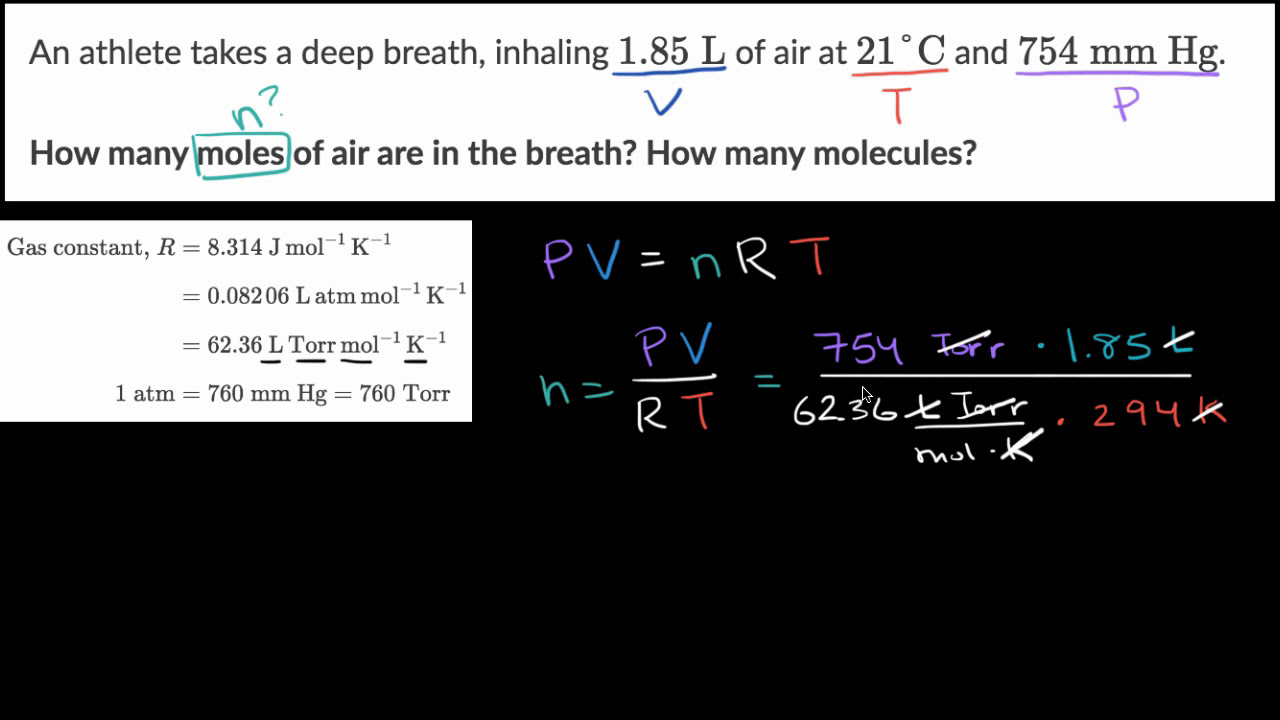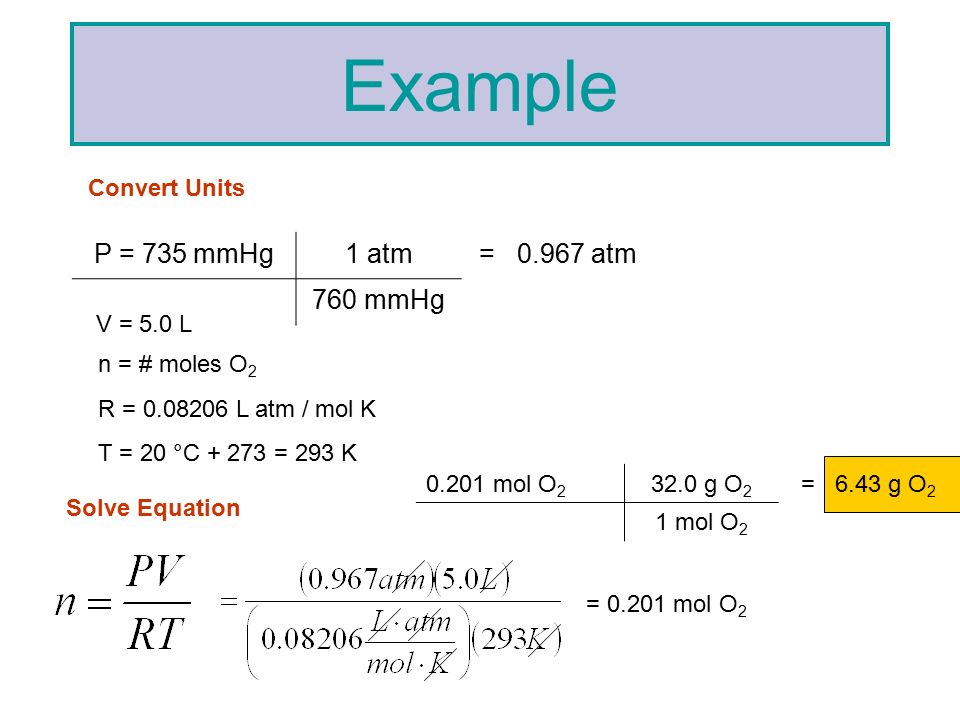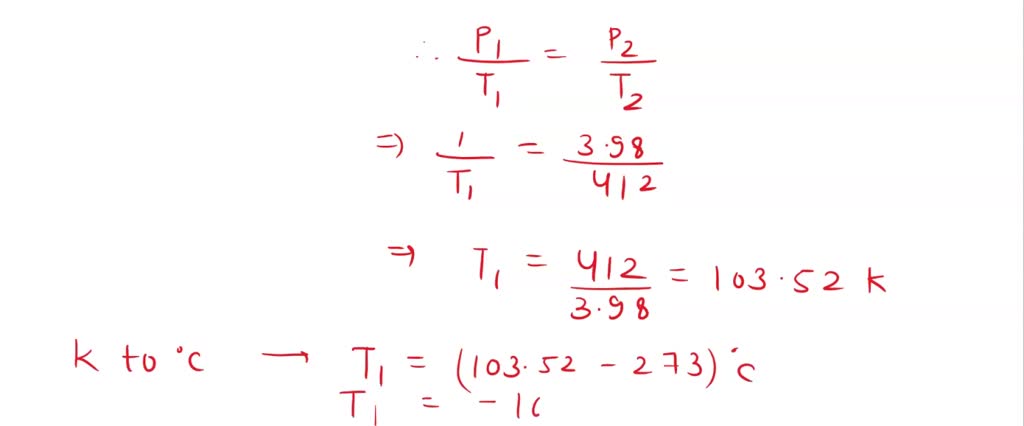
SOLVED: An ideal gas is heated at constant volume During this process the pressure increased from 1.00 atm to 3.98 atm. If the final temperature of the gas is found to be

An ideal gas at 0.80 atmospheres and 87°C occupies 0.450 liter. How many moles are in the sample? - YouTube

Ideal Gas Law Calculator (Pressure–Volume–Temperature–Amount) • Thermodynamics — Heat • Online Unit Converters
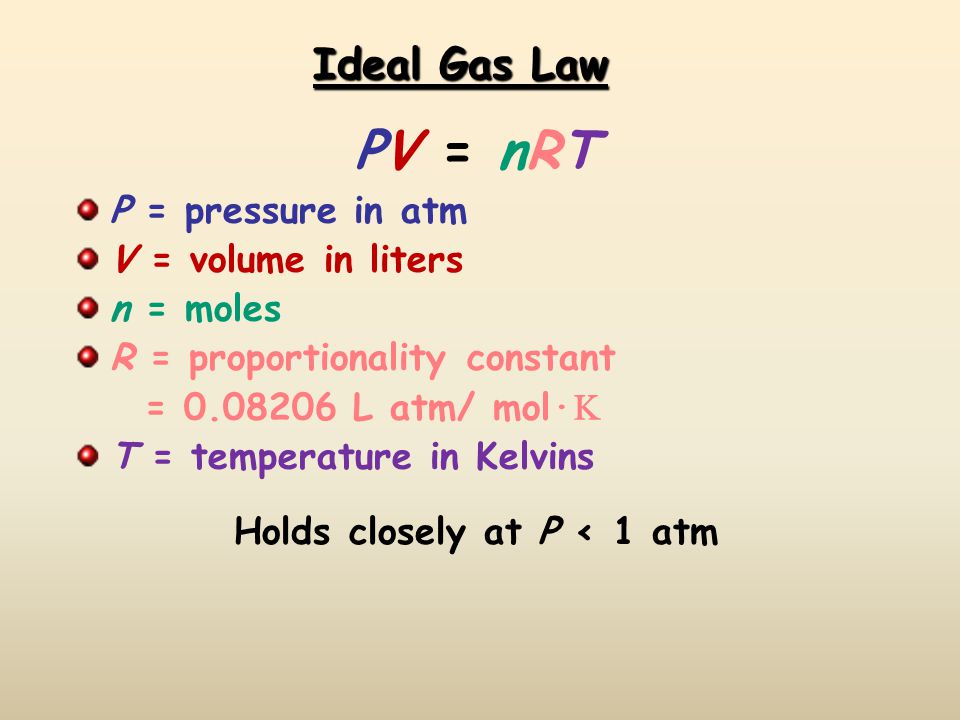
If I have 21 moles of gas held at a pressure of 3800 torr and a temperature of 627°C what is the volume of the gas? | Socratic

molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

OpenStax College Physics Solution, Chapter 13, Problem 28 (Problems & Exercises) | OpenStax College Physics Answers

Calculate the temperature of 4.0 moles of a gas occupying 5 dm^3 at 3.32 bar (R = 0.083 bar dm^3 K^-1 mol^-1) .