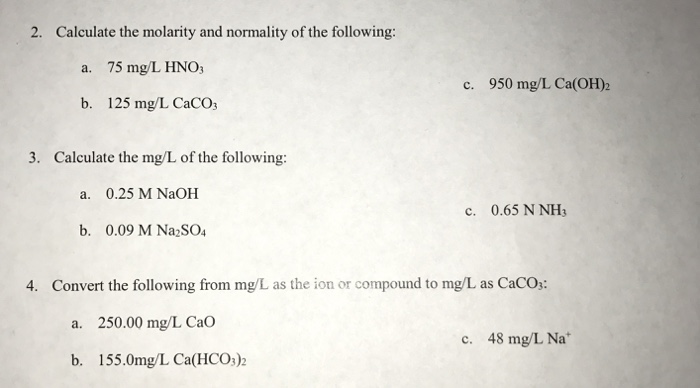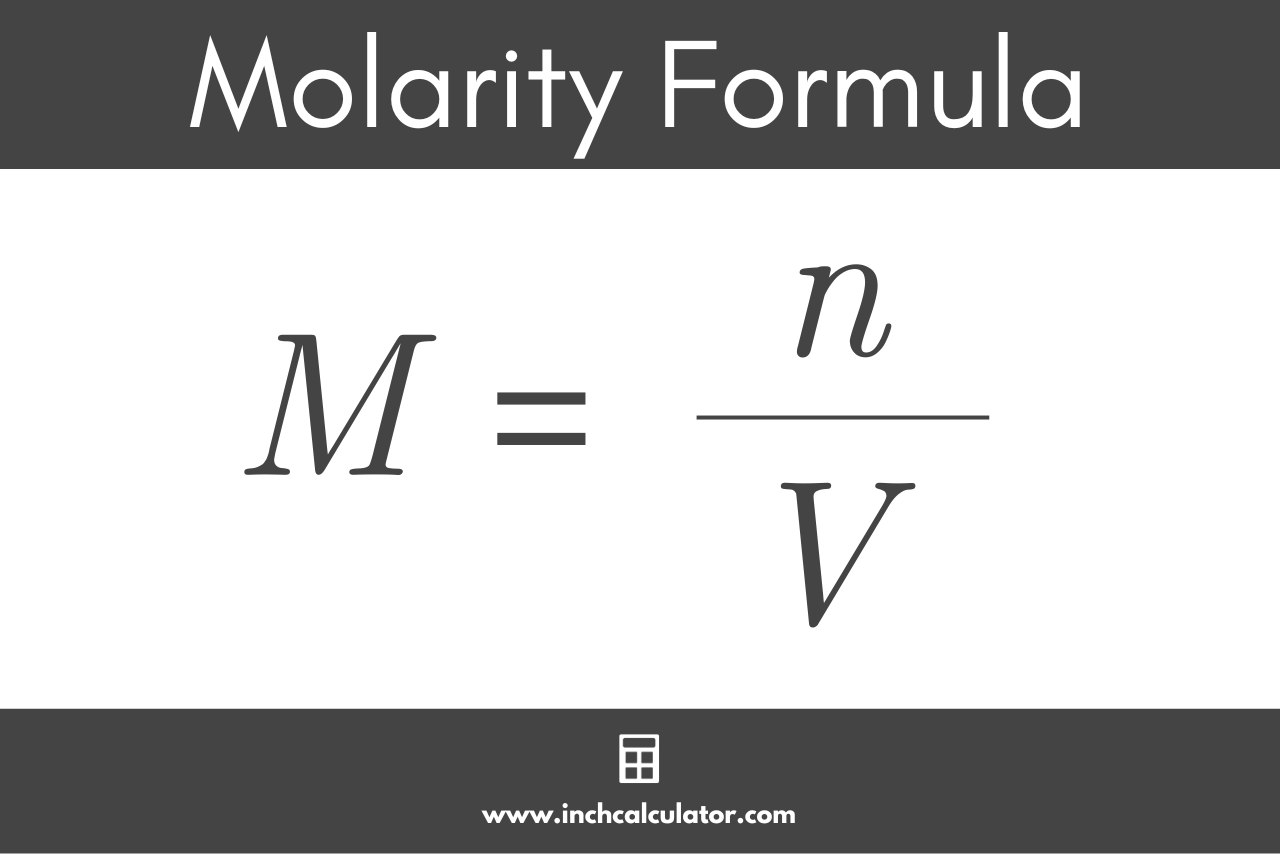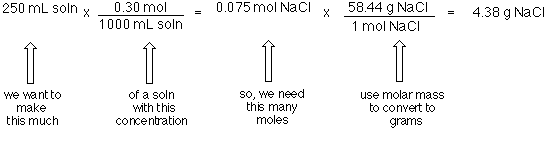Question Video: Calculating the Molar Concentration of Mg(OH)₂ Using Data from a Titration Experiment | Nagwa
What is the molarity of a solution that contains 50.0 g of Mg(NO_3)_2 per 225 mL of solution? | Socratic

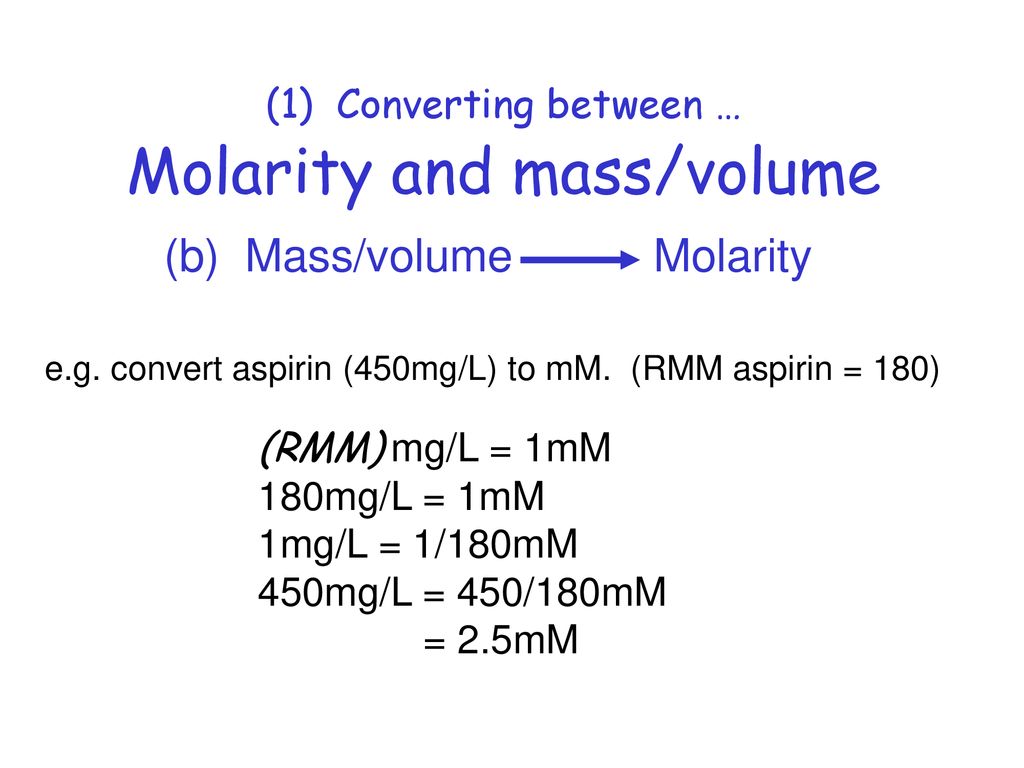
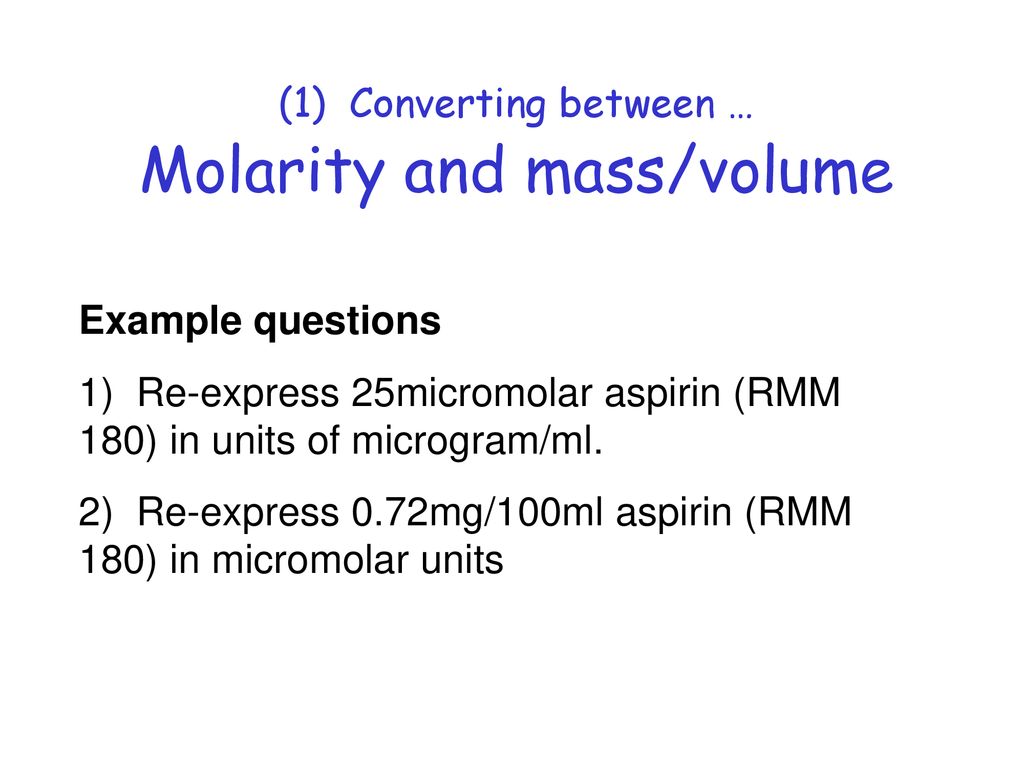
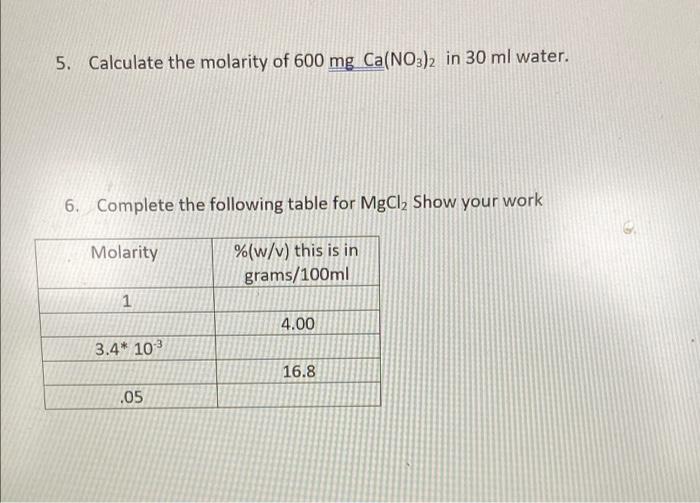
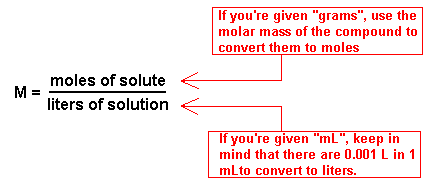
Calculating molarity units molar concentration of solutions practice questions on molarity how to make up a standard solution how to determine solubility gcse chemistry igcse KS4 science A level GCE AS A2

Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry - YouTube

CALCULATIONS I. Reconstituting Cytokines/Growth Factors Need To Supplement Cultures With Recombinant Growth factors/Cytokines Issues To Consider –Recombinant. - ppt download

I have H2O2 of molecular wt 34.01gm and 30% w/v. What does it mean that I am not getting it and I want to prepare 0.1M solution, how can i? | ResearchGate